
Thermodynamicist is a C# program made to calculate basic thermodynamic properties of chemical systems. Many equations used in this code, as well as the built-in data tables, are pulled directly from Sandler’s Chemical, Biochemical, and Engineering Thermodynamics (5th edition). When used, sources are marked in the code with a comment.
The spiritual predecessor to this program was written in MATLAB by Professor Ali Mohraz from the University of California, Irvine. I originally began writing this program in Java but was convinced to switch to C#/.NET for better UI and performance. Construction began around the beginning of May 2022 and had successfully implemented all features that the original MATLAB code had included in late June 2022.
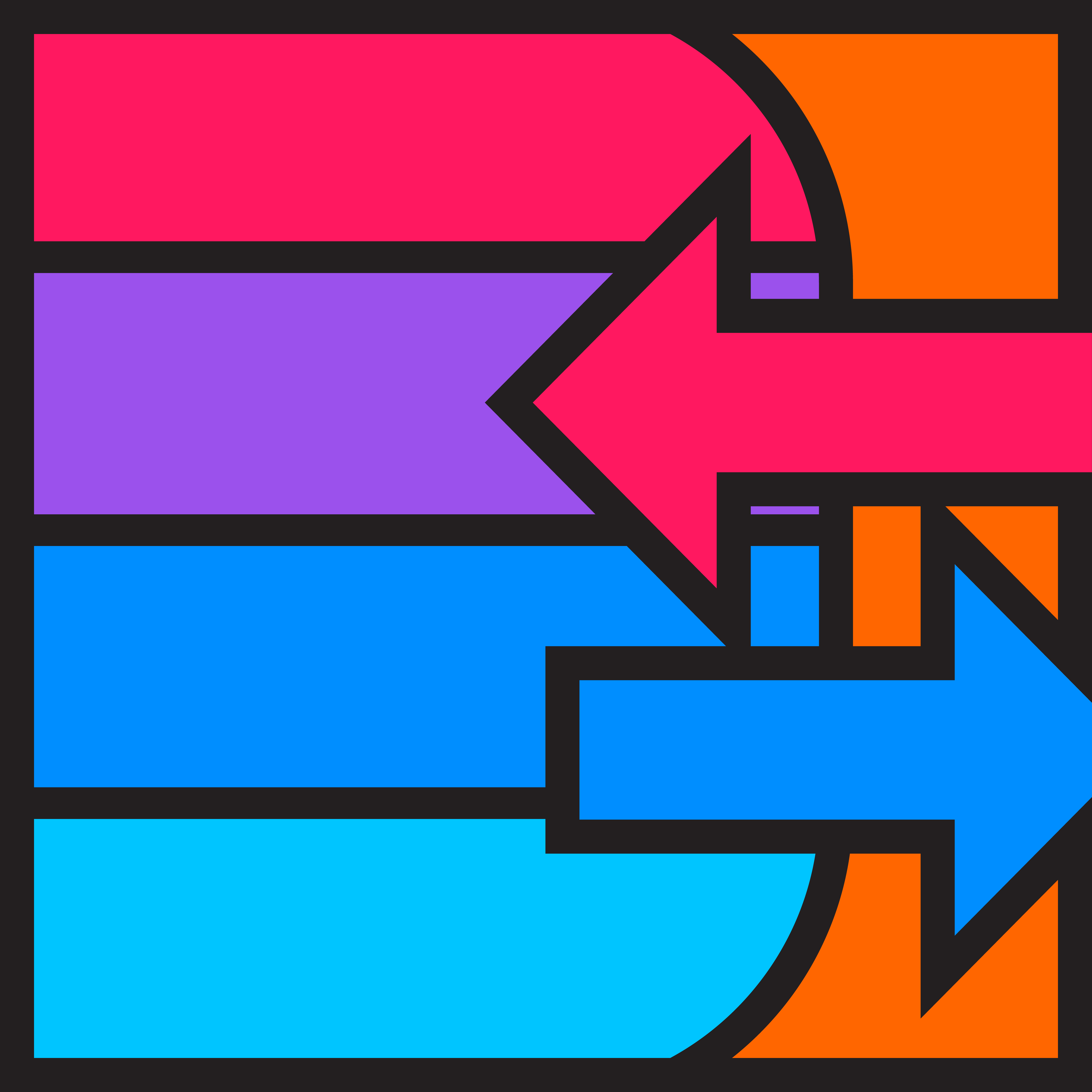
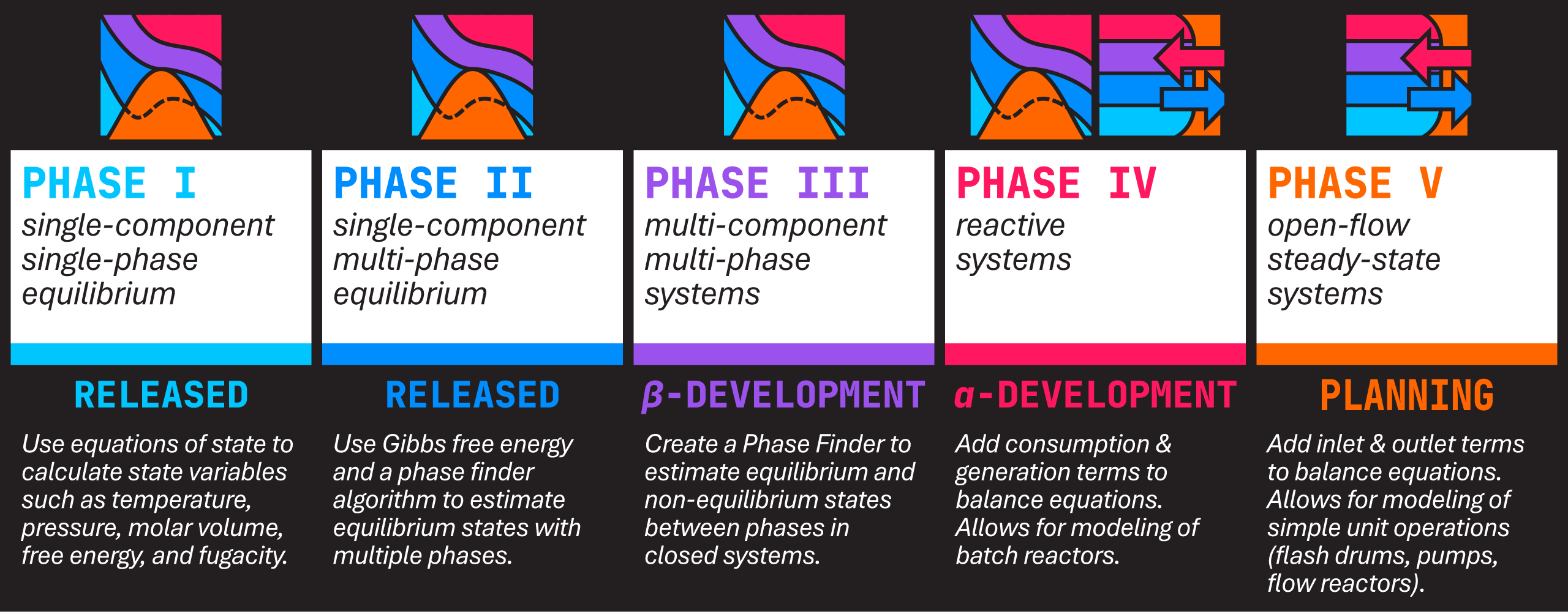
Development Plan
Features
Thermodynamicist provides users with the ability to…
- Do thermodynamic calculations using common equations of state, such as the van der Waals or Peng-Robinson equation of state.
- Estimate entropies and heats of reaction from formation enthalpies.
- Estimate pure component state variables, such as fugacity, molar volumes, and enthalpy.
- Estimate enthalpy, entropy, and free energies with respect to an ideal reference state (currently defaults to 25°C and 100 kPa).
- Determine present phases in single-component equilibrium.
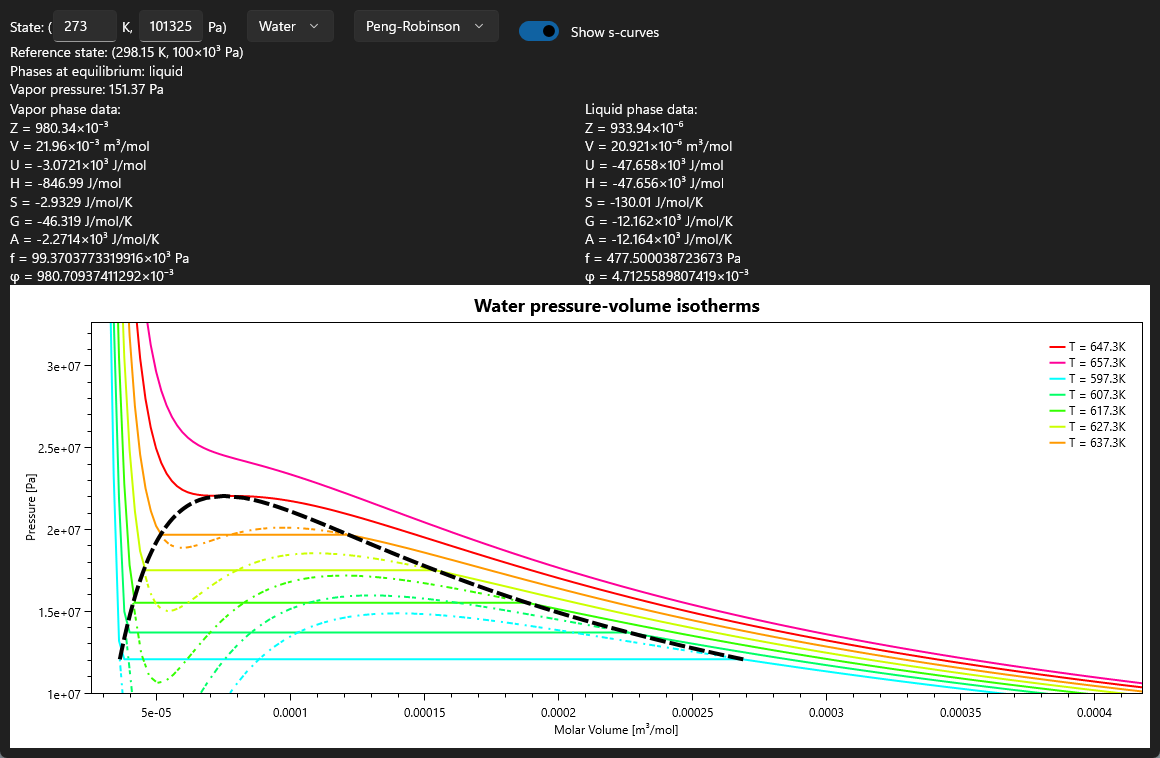
- Calculate vapor pressure and plot isotherms.
Code Structure
In mid-July 2022, the first dynamic inputs were added to the UI, allowing users to select a temperature, pressure, and chemical species to be used for calculations. After a few months of being kept busy with summer classes, the first plotting output (for pressure-volume isotherms) was added in late September 2022. By November, this plot could correct the S-curve behavior exhibited by equations of state using the vapor pressure.
In early 2025, the project entered into Phase IV of development with the addition of the Reaction class and related handling for calculating heats and enthalpy of reaction. This phase marks the first overt steps towards turning Thermodynamicist into a process simulator.
